 |
New York (AFP) April 22, 2008 A contaminated blood thinner from China linked to 81 deaths in the United States is present in drug supplies in 11 countries, the New York Times said Tuesday, citing federal regulators. The US Food and Drug Administration Monday sent a warning letter to Changzhou SPL, the Chinese plant identified as the source of contaminated heparin sold by Baxter International in the United States, the Times said. In its letter the FDA said the plant used unclean tanks to make heparin, accepted raw materials from an unacceptable vendor and had no way to remove impurities, according to the Times. The FDA has identified 12 Chinese companies that have supplied contaminated heparin to Australia, Canada, China, Denmark, France, Germany, Italy, Japan, the Netherlands, New Zealand and the United States, the report said. But it does not know the original source of the contamination or where it entered the supply chain, the Times said. The FDA has identified the contaminant as oversulfated chondroitin sulfate, a "cheap fake additive" that can be detected only through sophisticated testing, the paper said. A Chinese official disputed the assertion the blood thinner has caused death, but conceded that heparin produced in China contained a contaminant. "We don't have a strong evidence to show that it is heparin or its contaminant that caused the problem," Ning Chen, second secretary of the Chinese embassy, told the Times. He said the illnesses associated with heparin had occurred only in the United States, and insisted Chinese inspectors should be allowed to inspect the US plant where the final stage of the drug's production took place. German regulators have also reported a rash of illnesses in patients using heparin, FDA official Janet Woodcock said. "Heparin should not be contaminated, regardless of whether or not that contamination causes acute adverse events," Woodcock was quoted as saying. "We are fairly confident based on the biological information that we have had that this contaminant is capable of triggering these adverse reactions." These include allergic or hypersensitivity-type reactions, with symptoms including low blood pressure, shortness of breath, nausea, vomiting, diarrhea, and abdominal pain, according to the FDA's website. The FDA launched an investigation after seeing a spike in deaths reported after heparin administered between November and February. In 2006, 55 deaths were reported after a patient used heparin. A variety of underlying medical conditions were involved, and only three of the deaths listed allergic reactions or low blood pressure among the medical events preceding death, the FDA said. From January 2007 to the present, 131 deaths were reported, including 81 involving allergic reactions or hypotension, prompting a heparin recall and the investigation. The FDA on March 5 said it had detected an unidentified contaminant in heparin injections sold by Baxter International. It was determined that most of the active ingredients in the drug came from a plant in Changzhou, eastern China working with Wisconsin-based Scientific Protein Laboratories, which supplies Baxter. Community Email This Article Comment On This Article Related Links Hospital and Medical News at InternDaily.com
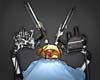 Baltimore MD (SPX) Mar 28, 2008
Baltimore MD (SPX) Mar 28, 2008A medical student places a chest tube in a patient lying on an operating table, while another student conducts a colonoscopy. Everything is just as it would be in a real OR or treatment room, except that the patients won't be harmed or complain if mistakes are made - they're robots. |
|
| The content herein, unless otherwise known to be public domain, are Copyright 1995-2007 - SpaceDaily.AFP and UPI Wire Stories are copyright Agence France-Presse and United Press International. ESA Portal Reports are copyright European Space Agency. All NASA sourced material is public domain. Additional copyrights may apply in whole or part to other bona fide parties. Advertising does not imply endorsement,agreement or approval of any opinions, statements or information provided by SpaceDaily on any Web page published or hosted by SpaceDaily. Privacy Statement |